

(Note the tubes across the title of this page - they are the set of samples set up for this experiment). The darker colored the solution, the more light it absorbs. As the amount of protein in a solution increases, the color of the solution becomes darker. The more protein present, the darker blue the indicator turns. The dye starts out reddish brown, but turns blue when it reacts with protein. We started by mixing known amounts of a protein (albumin) with a dye indicator called Coomassie Blue. It can tell you how much light is passing through a solution ( transmittance) or how much light is being absorbed by a solution ( absorbance). Spectrophotometry and Protein ConcentrationĪ spectrophotometer is a machine that measures light quantity.
Spectrophotometry can be used for determining the amount of many types of substances in solution, from living bacteria cells to DNA fragments. How can you test an unknown solution to determine its solute concentration? The method described below relies upon standard lab equipment, and while we used it to determine protein concentration, it has a wide variety of applications. Many online news and information platforms allow us to vote on the content, noting what we click on, what we like, and what we ignore.Water Chemistry I: Dilutions & Solute Concentration.Public Perception of Diseases – Thought Response – 200 Words.As we provide this information, self-segmentation can occur over Many online news and information platforms allow us to vote on the content, noting what we click on, what we like, and what we ignore.Clinical Field Experience B: Schoolwide Budgetary Needs.
How to do dilution series full#

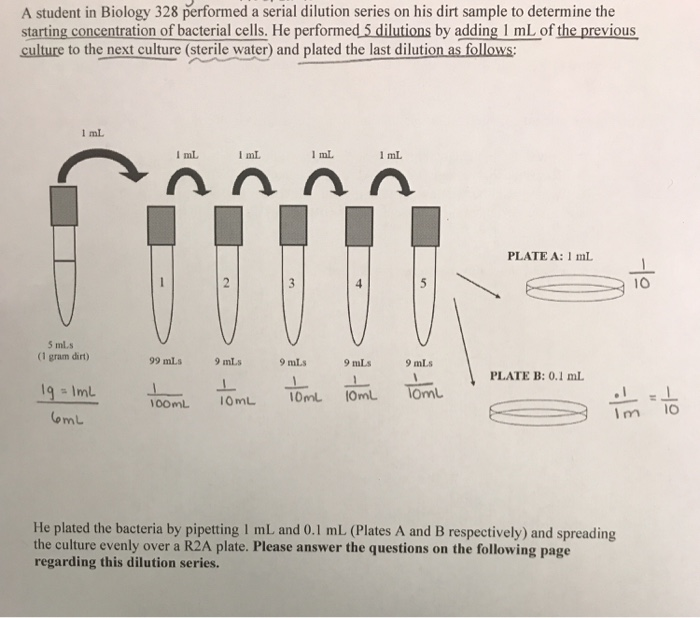
Repeat the process until you have four tubes. Then transfer 0.2 mL from Tube 2 to 3.8 mL of diluent in Tube 3 and mix. You would transfer 0.2 mL from Tube 1 to 3.8 mL of diluent in Tube 2 and mix. So you multiply each successive dilution by the dilution factor.
How to do dilution series serial#
Remember that serial dilutions are always made by taking a set quantity of the initial dilution and adding it successively to tubes with the same volume. If you did the above dilution four times, what would be the final dilution factor? What is the dilution factor if you add 0.2 mL of a stock solution to 3.8 mL of diluent? The dilution factor or the dilution is the initial volume divided by the final volume.įor example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution, In serial dilutions, you multiply the dilution factors for each step. You multiply the original concentration by the dilution factors for each dilution.Ī serial dilution is any dilution in which the concentration decreases by the same factor in each successive step.


 0 kommentar(er)
0 kommentar(er)
